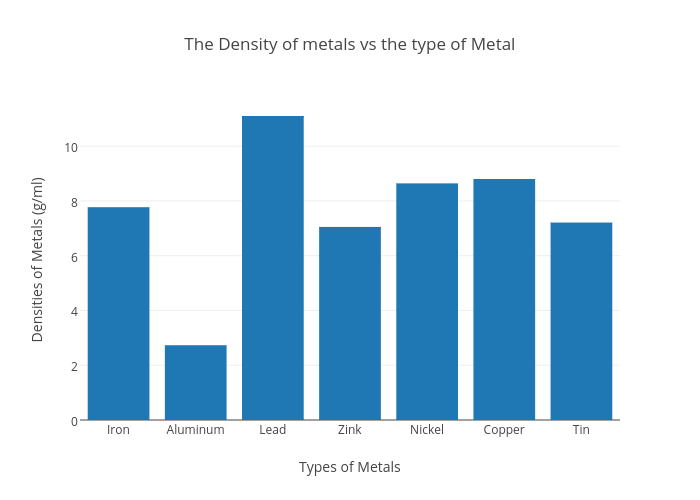
Density Of Metals Chart . Using the table above, what is this substance? Use the table above to identify the metal.
Density from www.eoas.ubc.ca
Density is defined as the mass per unit volume. It can be measured in grams per cubic centimeter (g/cm3). 97 rows density name symbol # 0.0899 g/l:
Density But just merely knowing the name of each and every one of the elements is not enough. Mathematically, density is defined as mass divided by volume: The letter “d” is often used. It can be measured in grams per cubic centimeter (g/cm3).
Source: Density is defined as the mass per unit volume. The chart below describes the various specific gravities of architectural metals, which range from the lightness of titanium and aluminum to the heavy density of lead and gold metals. Metal and alloy densities vary significantly and it depends on the metal element. 97 rows density name symbol # 0.0899 g/l: Using.
Source: www.blendspace.com 127 rows the density of some common metals, metallic elements and alloys are indicated in. For example, beryllium has a specific gravity (sg) of 1.84 (1840 kg/cu.m) (see table below) as specific gravity is just a comparison, it can be applied across any units. Densities of metals and elements table. The metal is _____ is palladium. For kg/m 3, multiply.
Source: www.researchgate.net 97 rows density name symbol # 0.0899 g/l: For kg/m 3, multiply density in lb/in. Here's a table of densities of common substances, including several gases, liquids, and solids. Density is defined as a object’s mass per volume. For density in lb/ft 3, multiply lb/in.
Source: www.quora.com To calculate density, you need to know the object’s mass (in grams or pounds) and its volume (size). A substance has a mass of 1370.3 grams (g) and a volume of 71 cubic centimeters (cm3). Here's a table of densities of common substances, including several gases, liquids, and solids. The letter “d” is often used. Metal / element or alloy.
Source: intertechonline.blogspot.com The letter “d” is often used. The metal is _____ is palladium. Metal and alloy densities vary significantly and it depends on the metal element. Density is defined as a object’s mass per volume. The symbol most often used for density is ρ, although the latin letter d can also be used.
Source: www.pinterest.com Metal and alloy densities vary significantly and it depends on the metal element. For density in lb/ft 3, multiply lb/in. Here we collect the metal strength chart (tensile, yield strength, hardness, and density included) and. For this reason, the table lists density from lowest to highest. But just merely knowing the name of each and every one of the elements.
Source: www.theworldmaterial.com When we say that ice is less dense than water, we mean that the water molecules are more tightly packed when they are in the liquid state. For example, beryllium has a specific gravity (sg) of 1.84 (1840 kg/cu.m) (see table below) as specific gravity is just a comparison, it can be applied across any units. Is the symbol for.
Source: sites.google.com To calculate density, you need to know the object’s mass (in grams or pounds) and its volume (size). Is the symbol for mass. Calculating density density of common metals copper 8.96 g/cm 3 gold 19.32 g/cm3 iron 7.87 g/cm3 lead 11.36 /cm3 1. 127 rows the density of some common metals, metallic elements and alloys are indicated in. The density.
Source: chart-studio.plotly.com Strength is a critical factor in metal uses, for example, some applications require stronger aluminum parts, while some products need high steel hardness or yield strength of steel, this may determine the selection of cnc machining material or product design. Density is defined as the mass per unit volume. The chart below describes the various specific gravities of architectural metals,.
Source: www.eoas.ubc.ca Density is defined as the mass per unit volume. Density 10 young’s modulus 11. Ρ = m v {\displaystyle \rho ={\frac {m}{v}}} where ρ is the density, m is the mass, and v is the volume. Calculating density density of common metals copper 8.96 g/cm 3 gold 19.32 g/cm3 iron 7.87 g/cm3 lead 11.36 /cm3 1. To convert between ib/ft.
Source: chart-studio.plotly.com Density is defined as a object’s mass per volume. The density (specific mass) of a substance is its mass per unit volume. But just merely knowing the name of each and every one of the elements is not enough. 127 rows the density of some common metals, metallic elements and alloys are indicated in. To convert between kg/m 3 and.
Source: www.chegg.com Table shows density of selected gases, solids and liquids. For density in lb/ft 3, multiply lb/in. In some cases, density is loosely defined as its weight per unit. The density, of a substance is its mass per unit volume. Is the symbol for mass.
Source: www-materials.eng.cam.ac.uk Densities of metals and elements table. It is a measure of how tightly packed the atoms of a substance are. For density in lb/ft 3, multiply lb/in. The chart below describes the various specific gravities of architectural metals, which range from the lightness of titanium and aluminum to the heavy density of lead and gold metals. Below is the density.
Source: weldguru.com Knowing the periodic table is key for most scientists. The density, of a substance is its mass per unit volume. Volume and then the density. The periodic chart sorted by: Uniaxial tensile response of selected metals and polymers 15 iii.
Source: chart-studio.plotly.com The density (specific mass) of a substance is its mass per unit volume. For this reason, the table lists density from lowest to highest. The density of pure water is also 62.4 lbs/cu.ft (pounds per cubic foot) and if we know that a sample of alumimium has a sg of 2.5 then we can calculate that its density is 2.5.
Source: sciencetrends.com The density (specific mass) of a substance is its mass per unit volume. Below is a metal density table for common metals. A substance has a mass of 1370.3 grams (g) and a volume of 71 cubic centimeters (cm3). The equation for material density is: Is the symbol for density.
Source: www.azahner.com Metal and alloy densities vary significantly and it depends on the metal element. To convert between kg/m 3 and ib/ft 3, then we need to multiply by 0.062428.therefore, 1 kg/m 3 equals 0.062428 ib/ft 3. When we say that ice is less dense than water, we mean that the water molecules are more tightly packed when they are in the.
Source: www.chegg.com The density, of a substance is its mass per unit volume. Density table of metals (iron, steel brass, aluminium) and alloys. Density 10 young’s modulus 11. The letter “d” is often used. The density of pure water is also 62.4 lbs/cu.ft (pounds per cubic foot) and if we know that a sample of alumimium has a sg of 2.5 then.
Source: www.researchgate.net Juan ramos on january 16, 2018 3 comments ! The density, of a substance is its mass per unit volume. Use the table above to identify the metal. 97 rows density name symbol # 0.0899 g/l: The symbol most often used for density is ρ, although the latin letter d can also be used.
Source: www.pwbeck.com.au The symbol most often used for density is ρ, although the latin letter d can also be used. Mathematically, density is defined as mass divided by volume: To calculate density, you need to know the object’s mass (in grams or pounds) and its volume (size). The periodic chart sorted by: The letter “d” is often used.