Ground State Electron Configuration Chart . There are 118 elements in the periodic table. Promotion of an electron to a higher energy level (forming b*) let's take boron as a normal example.
Ground State Electron Configuration: Definition & Example - Video & Lesson Transcript | Study.com from study.com
Which atom in the ground state has the same electron configuration as a calcium ion ca2+? The ground state for ge is ge:[ar]3d10 4s2 4p2, so for ge2+, it would be ge2+:[ar]3d10 4s2, since two electrons are removed. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus.
Ground State Electron Configuration: Definition & Example - Video & Lesson Transcript | Study.com When we talk about the ground state electronic configuration firstly, it is important. How do you excite an electron? The electron configuration 1s22s22p63s23p2 is the element silicon. There are 118 elements in the periodic table.
Source: www.youtube.com 119 rows the electron configuration shows the distribution of electrons into. When we talk about the ground state electronic configuration firstly, it is important. The ground state electron configuration of ground state gaseous neutral calcium is [ar]. However, the quantum mechanical states of a single electron can also be described by a term symbol. The electron configuration 1s22s22p63s23p2 is the.
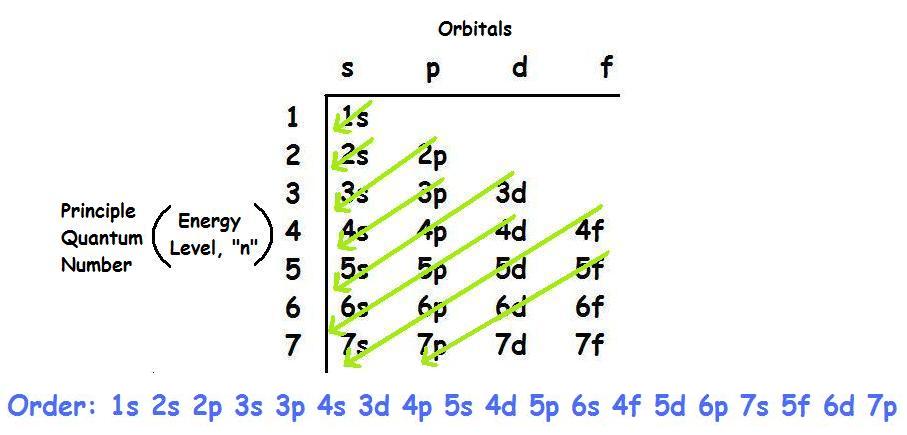
Source: knowledgebin.org The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration. Ground state electron configuration of sodium (na): Energy—ground state and excited states. When an atom or ion receives electrons into its orbitals, the orbitals fill up in thefollowing order: 119 rows electron configuration chart of all elements (full chart) november.
Source: terpconnect.umd.edu Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. Electron configuration practice chemistry name : The most stable arrangement or the lowest energy is referred to as the ground state configuration. To determine the electron configuration of a particular atom, start at the.
Source: www.thoughtco.com Learn more about the definition of. The electron configuration of the chemical element describes the ground state, i.e. The electron configuration of calcium ion, ca2+ is 2, 8, 8. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. However, the quantum mechanical states.
Source: studyqueries.com The ground state electron configuration of ground state gaseous neutral oxygen is [he]. How do you excite an electron? When the atom is in excited state, one or more electrons go to a higher energy state, so electron configuration of. The ground state for ge is ge:[ar]3d10 4s2 4p2, so for ge2+, it would be ge2+:[ar]3d10 4s2, since two electrons.
Source: www.webelements.com Promotion of an electron to a higher energy level (forming b*) let's take boron as a normal example. When we talk about the ground state electronic configuration firstly, it is important. Write a ground state electron configuration for these ions. The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration..
Source: www.youtube.com The most stable arrangement or the lowest energy is referred to as the ground state configuration. The ground state electronic configurations of the 0, +1, and +2 oxidation states of the element scandium (sc) provide a really good example for this argument. 2p 4 and the term symbol is 3 p 2. Electron configuration practice chemistry name : That means,.
Source: socratic.org An electron configuration calculator provides abbreviated electron configuration, standard state, atomic mass,. The ground state electronic configurations of the 0, +1, and +2 oxidation states of the element scandium (sc) provide a really good example for this argument. The numbers with s come first following p and d and f. The ground state electron configuration of ground state gaseous neutral.
Source: study.com Learn more about the definition of. 4s2 and the term symbol is 1s0. There are 118 elements in the periodic table. Remember that ions have a change in the total number of electrons (positive have lost electrons and negative have gained). By following the diagonal arrow method you can easily write down the ground state electron configuration of sodium (na).
Source: periodictable.me There are 118 elements in the periodic table. When writing down the configuration remember the order of s, p, d, f. 119 rows electron configuration chart of all elements (full chart) november 1,. The electron configuration 1s22s22p63s23p2 is the element silicon. The state in which all electrons have the lowest possible energy.
Source: www.quora.com 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 8s Ground state means that all of the lowest possible energy levels (up to the proper number of electrons for the element) are filled. The numbers with s come first following p and d and f. The electron configuration 1s22s22p63s23p2 is.
Source: study.com The ground state for ge is ge:[ar]3d10 4s2 4p2, so for ge2+, it would be ge2+:[ar]3d10 4s2, since two electrons are removed. That means, it has lost three electrons from its electron configuration, and therefore loses both of its 4s electrons, and one 3d electron, thus leaving a. When the atom is in excited state, one or more electrons go.
Source: docbrown.info From the source of science notes: 2p 4 and the term symbol is 3 p 2. The electron configuration 1s22s22p63s23p2 is the element silicon. Fe 13 che1 14 ni2+ 15. When we talk about the ground state electronic configuration firstly, it is important.
Source: chem.libretexts.org The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration. 119 rows electron configuration chart of all elements (full chart) november 1,. Electron configuration practice chemistry name : When an atom or ion receives electrons into its orbitals, the orbitals fill up in thefollowing order: 2p 4 and the term.
Source: www.orangatame.com The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration. By following the diagonal arrow method you can easily write down the ground state electron configuration of sodium (na) 1s 2 2s 2 2p 6 3s 1. 107 rows updated on february 01, 2021 the electron configuration of an atom..
Source: study.com The numbers with s come first following p and d and f. 4s2 and the term symbol is 1s0. For oxygen, z=8, and thus o2− has a configuration of: That means, it has lost three electrons from its electron configuration, and therefore loses both of its 4s electrons, and one 3d electron, thus leaving a. The ground state for ge.
Source: study.com Now, we need to find the electron configuration for a nickel (iii) cation. However, the quantum mechanical states of a single electron can also be described by a term symbol. The ground state for ge is ge:[ar]3d10 4s2 4p2, so for ge2+, it would be ge2+:[ar]3d10 4s2, since two electrons are removed. When writing down the configuration remember the order.
Source: chem.libretexts.org The ground state for ge is ge:[ar]3d10 4s2 4p2, so for ge2+, it would be ge2+:[ar]3d10 4s2, since two electrons are removed. The answer is (1) ar. When we talk about the ground state electronic configuration firstly, it is important. Write a ground state electron configuration for each neutral atom. When the atom is in excited state, one or more.
Source: courses.lumenlearning.com The electron configuration of calcium ion, ca2+ is 2, 8, 8. For oxygen, z=8, and thus o2− has a configuration of: There are 118 elements in the periodic table. How do you excite an electron? The numbers with s come first following p and d and f.
Source: socratic.org Write a ground state electron configuration for these ions. Electron configuration practice chemistry name : How do you excite an electron? Learn more about the definition of. The ground state electron configuration of ground state gaseous neutral oxygen is [he].