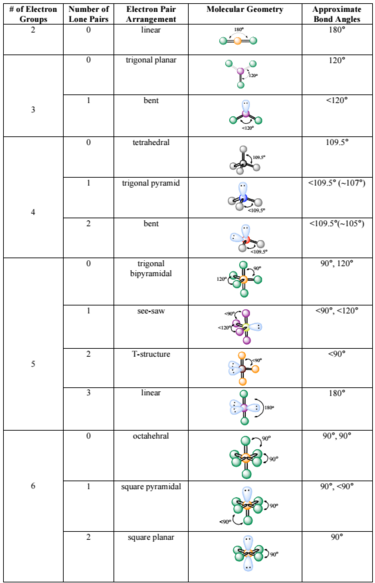
Molecular And Electron Geometry Chart . The relationship between the number of. 3 electron groups 0 lone pairs 3 bonds.
Molecular Geometry Chart With Angles - Tutordale from www.tutordale.com
Molecular geometry worksheet pdf.molecular geometry ch. 05 october 2020 * if you want to update the article please login/register. The valence shell electron pair repulsion model focuses on bonding and nonbonding electron pairs present in the outermost shell of atom that connect with two or more other atoms.
Molecular Geometry Chart With Angles - Tutordale Lewis electron dot diagrams and sketches of molecules may be helpful as part of your explanations. 3electron groups 1 lone pair 2 bonds. Chang, 8th edition, chapter 10 s. The relationship between the number of.
Source: The molecule adopts whichever 3d geometry minimized this repulsion. The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Choose the one alternative that best completes the statement or answers the question. Chang, 8th edition, chapter 10 s. To predict molecular shape, assume the valence electrons repel each other.
Source: www.pinterest.com Chang, 8th edition, chapter 10 s. 26 rows table summarizing molecular geometries. Electron domain geometry chart, molecular geometry chart molecular geometry chart 22012653, 1 electron domain molecular geometry electron domain, molecular geometry boundless chemistry, electron domain google search education, oh education Teach your students about vsepr theory and molecular geometry using this great detailed worksheet this worksheet guides students through.
Source: The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Electron domain geometry chart, molecular geometry chart molecular geometry chart 22012653, 1 electron domain molecular geometry electron domain, molecular geometry boundless chemistry, electron domain google search education, oh education A molecular geometry chart is a collection of rules on how molecules and electrons will connect.
Source: sansona.github.io Predict the geometry of the molecule from the electrostatic repulsions between the. Piepho, fall 2005 molecular geometry summary sheet shaded Molecular geometry worksheet pdf.molecular geometry ch. Molecular and electron geometry chart summarized by plexpage. Electron and molecular geometry chart summarized by plexpage.
Source: www.tutordale.com The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Besides this, the lewis structure helps with determining the hybridization of the molecule. Ax n e n > = 1. Ab n e m # of electron regions electron geometry # of bonding regions # of nonbonding. The relationship between the number of.
Source: www.sigmaaldrich.com Electron and molecular geometry chart summarized by plexpage. Ab n e m # of electron regions electron geometry # of bonding regions # of nonbonding. 05 october 2020 * if you want to update the article please login/register. When lone electron pairs exist, they affect the molecular geometry, even if their location is not directly specified in the molecular geometry.
Source: socratic.org The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Pre ap chemistry electronic and molecular geometry worksheet hw 2a. The lewis structure helps with understanding how electrons are distributed within a compound along with its molecular geometry. 3 electron groups 0 lone pairs 3 bonds. The first molecular geometry theory (vsepr):
Source: www.pinterest.com I m f s 1 ocl2 2 hf 3 chcl3. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). The valence shell electron pair repulsion model focuses.
Source: www.speedytemplate.com 4 electron groups 0 lone pairs 4. These molecules are usually polar, with the exception of. Choose the one alternative that best completes the statement or answers the question. Lewis electron dot diagrams and sketches of molecules may be helpful as part of your explanations. 05 october 2020 * if you want to update the article please login/register.
Source: pdfsimpli.com Electron domain geometry chart, molecular geometry chart molecular geometry chart 22012653, 1 electron domain molecular geometry electron domain, molecular geometry boundless chemistry, electron domain google search education, oh education Molecular geometry van koppen/offen procedure: These molecules are usually polar, with the exception of. The lewis structure helps with understanding how electrons are distributed within a compound along with its molecular.
Source: www.coursehero.com In fact, they are actually more repulsive. Even though the lone pairs cannot be seen, they still repel the bonding pairs of electrons. Electron and molecular geometry chart summarized by plexpage. Choose the one alternative that best completes the statement or answers the question. Pre ap chemistry electronic and molecular geometry worksheet hw 2a.
Source: chem.libretexts.org The first molecular geometry theory (vsepr): Some other examples shown on the vsepr chart are sulfur hexafluoride, sf. Products of moments of inertia; The lewis structure helps with understanding how electrons are distributed within a compound along with its molecular geometry. Ab n e m # of electron regions electron geometry # of bonding regions # of nonbonding.
Source: www.pinterest.com The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. 4 electron groups 0 lone pairs 4. Pre ap chemistry electronic and molecular geometry worksheet hw 2a. The molecule adopts whichever 3d geometry minimized this repulsion. These molecules are usually polar, with the exception of.
Source: www.bizzlibrary.com Besides this, the lewis structure helps with determining the hybridization of the molecule. Electron domain geometry chart, molecular geometry chart molecular geometry chart 22012653, 1 electron domain molecular geometry electron domain, molecular geometry boundless chemistry, electron domain google search education, oh education To predict molecular shape, assume the valence electrons repel each other. Some other examples shown on the vsepr.
Source: courses.lumenlearning.com 3 electron groups 0 lone pairs 3 bonds. The valence shell electron pair repulsion model focuses on bonding and nonbonding electron pairs present in the outermost shell of atom that connect with two or more other atoms. The valence shell electron pair repulsion model focuses on bonding and nonbonding electron pairs present in the outermost shell of atom that connect.
Source: courses.lumenlearning.com The valence shell electron pair repulsion model focuses on bonding and nonbonding electron pairs present in the outermost shell of atom that connect with two or more other atoms. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Electron domain geometry chart, molecular geometry chart molecular geometry chart 22012653,.
Source: www.reddit.com Products of moments of inertia; Molecular geometry worksheet pdf.molecular geometry ch. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Besides this, the lewis structure helps with.
Source: www.chemistrysteps.com In fact, they are actually more repulsive. Once you know pcl 5 has five electron pairs, you can identify it on a vsepr chart as a molecule with a trigonal bipyramidal molecular geometry. 26 rows table summarizing molecular geometries. Terms in this set (37) 2 electron groups no lone pairs 2 bonds. 3 electron groups 0 lone pairs 3 bonds.
Source: www.templateroller.com The valence shell electron pair repulsion model focuses on bonding and nonbonding electron pairs present in the outermost shell of atom that connect with two or more other atoms. Molecular and electron geometry chart summarized by plexpage. I m f s 1 ocl2 2 hf 3 chcl3. Molecular shape and vsepr theory molecule total valence electrons lewis structure steric number.
Source: ontrack-media.net Chang, 8th edition, chapter 10 s. These molecules are usually polar, with the exception of. Some other examples shown on the vsepr chart are sulfur hexafluoride, sf. If one s and one p orbital hybridize, they form two sp hybrid orbitals. Besides this, the lewis structure helps with determining the hybridization of the molecule.