Pka Chart Amino Acids . Members of the basic family of amino acids, like lysine, will also exhibit three pka values. This video shows you how to use microsoft excel to find the equivalence points and calculate pka values for an amino acid titration.
Structures And Pka Values Of Amino Acids Used As Coating In The Study... | Download Scientific Diagram from www.researchgate.net
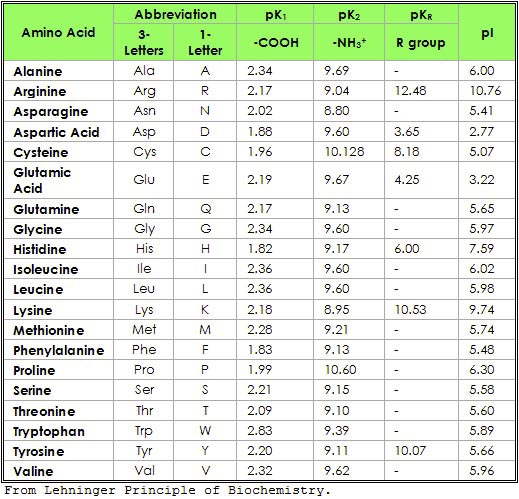
Aminocaproic acid competitively inhibits activation of plasminogen, thereby reducing conversion of plasminogen to plasmin (fibrinolysin), an enzyme that degrades fibrin clots as well as fibrinogen and other plasma proteins including the procoagulant factors v and viii. The pka of an amino acid depends upon its type, group and side chains. For example, when lysine is part of the carboxylic acid group, it has a pka of 2.18, compared to a pka of 8.95 when it is part of the amino group and 10.53 with side chains.
Structures And Pka Values Of Amino Acids Used As Coating In The Study... | Download Scientific Diagram Pka values of carboxylic acids, alcohols, phenols , amines. Pka data compiled by r. Aminocaproic acid is a synthetic lysine derivative with antifibrinolytic activity. See also boiling and melting point of.
Source: www.geneinfinity.org Members of the basic family of amino acids, like lysine, will also exhibit three pka values. Pka values of carboxylic acids, alcohols, phenols , amines. Ad aminozuren blends tegen betaalbare prijzen om je fitness naar een hoger niveau te tillen. This video shows you how to use microsoft excel to find the equivalence points and calculate pka values for an.
Source: www.quora.com Likewise, what does pka mean? The pka value given for the amino group on any amino acid specifically refers to the equilibrium between the protonated positive nitrogen and deprotonated neutral nitrogen. The ph at which the amino acid exists largely in a neutral, zwitterionic form (influenced by the nature of the sidechain) pk a~ 5 pk a~ 9 co2 r.
Source: www.researchgate.net This video shows you how to use microsoft excel to find the equivalence points and calculate pka values for an amino acid titration. 22 rows table of pkaand pi values the pkavalues and the isoelectronic point, pi, are given. Ad aminozuren blends tegen betaalbare prijzen om je fitness naar een hoger niveau te tillen. What is pka of amino acids?.
Source: www.pinterest.com Evans *values 14 for water and >35 for dmso were extrapolated using various methods. Pka values of carboxylic acids, alcohols, phenols , amines. Williams pka values index inorganic 2 phenazine 24 phosphates 3 pyridine 25 carboxylic acids 4, 8 pyrazine 26 aliphatic 4, 8 aromatic 7, 8 quinoline 27 phenols 9 quinazoline 27 alcohols and oxygen acids 10, 11 quinoxaline.
Source: www.researchgate.net Approximate pka chart of the functional groups: 22 rows table of pkaand pi values the pkavalues and the isoelectronic point, pi, are given. Aminocaproic acid is a synthetic lysine derivative with antifibrinolytic activity. Evans *values 14 for water and >35 for dmso were extrapolated using various methods. You'll never see a neutral nitrogen deprotonated to form a negative on an.
Source: www.chegg.com The number of pka values differentiates polar and nonpolar amino acids from charged amino acids. The pka of an amino acid depends upon its type, group and side chains. Pka values of carboxylic acids, alcohols, phenols , amines. Post navigation ← entropy calculation for ideal gas elements general physical properties : Members of the basic family of amino acids, like.
Source: www.sampletemplates.com Pka values of carboxylic acids, alcohols, phenols , amines. Their side chains contain nitrogen and resemble ammonia, which is a base. Bordwell pka table (acidity in dmso) organic chemistry info uw chemistry home uw organic chemistry home drawings produced with winplt. Terminal alkyne pka = 25 13. Dempsey (eds.), ionization constants of organic acids in solution, iupac chemical data series.
Source: www.semanticscholar.org 1116) 312 pi = pka x+ pka y 2 co2 c h3 h coh3n h 3n low ph co2 ch3 Their pka's are high enough that they tend to bind protons, gaining a. Approximate pka chart of the functional groups: Click to see full answer. Pka values of carboxylic acids, alcohols, phenols , amines.
Source: www.albert.io The ph at which the amino acid exists largely in a neutral, zwitterionic form (influenced by the nature of the sidechain) pk a~ 5 pk a~ 9 co2 r h h3n co + _ 2h r h h3n + co r h hn ho _ pka2 low ph high ph h3o _ pka1 table 27.2 (p. Aminocaproic acid is a.
Source: thepeacechallenge.blogspot.com However, due to the extra amino group, they will have only one pka in the acidic region and two pka values in the basic region. 1116) 312 pi = pka x+ pka y 2 co2 c h3 h coh3n h 3n low ph co2 ch3 Their pka's are high enough that they tend to bind protons, gaining a. Dempsey (eds.),.
Source: en.wikipedia.org Definitions of the acid dissociation constant and pka are given below the figures, together with the definition of some classes of organic acids. * first column (pka 1) = cooh * second column (pka 2) = nh 3 + *. The pka of an amino acid depends upon its type, group and side chains. Amine pka = 38 ‐40 14..
Source: www.pinterest.com In the table below, pk a1 and pk a2 is given together with boiling and melting point, density and molecular weight, as well as number of carbon, hydrogen and oxygen atoms in each molecule. This video shows you how to use microsoft excel to find the equivalence points and calculate pka values for an amino acid titration. These are arginine.
Source: www.reddit.com You'll never see a neutral nitrogen deprotonated to form a negative on an amino acid. 1116) 312 pi = pka x+ pka y 2 co2 c h3 h coh3n h 3n low ph co2 ch3 However, due to the extra amino group, they will have only one pka in the acidic region and two pka values in the basic region..
Source: www.masterorganicchemistry.com * first column (pka 1) = cooh * second column (pka 2) = nh 3 + *. The pka value given for the amino group on any amino acid specifically refers to the equilibrium between the protonated positive nitrogen and deprotonated neutral nitrogen. In the table below, pk a1 and pk a2 is given together with boiling and melting point,.
Source: www.researchgate.net Onze uithoudingssupplementen kunnen je helpen elke record te doorbreken, keer op keer 1116) 312 pi = pka x+ pka y 2 co2 c h3 h coh3n h 3n low ph co2 ch3 Members of the basic family of amino acids, like lysine, will also exhibit three pka values. Carboxylic acid pka = 4 ‐5 4. The pka of an amino.
Source: Williams pka values index inorganic 2 phenazine 24 phosphates 3 pyridine 25 carboxylic acids 4, 8 pyrazine 26 aliphatic 4, 8 aromatic 7, 8 quinoline 27 phenols 9 quinazoline 27 alcohols and oxygen acids 10, 11 quinoxaline 27 amino acids 12 special nitrogen compounds 28 peptides 13 hydroxylamines 28 nitrogen compounds 14. The pka value given for the amino group.
Source: wou.edu These are arginine (arg), lysine (lys), and histidine (his). If you cannot find the data that you need, please contact ivo.leito [at]ut.ee. In the table below, pk a1 and pk a2 is given together with boiling and melting point, density and molecular weight, as well as number of carbon, hydrogen and oxygen atoms in each molecule. Ad aminozuren blends tegen.
Source: www.chegg.com Ad aminozuren blends tegen betaalbare prijzen om je fitness naar een hoger niveau te tillen. Post navigation ← entropy calculation for ideal gas elements general physical properties : Bordwell pka table (acidity in dmso) organic chemistry info uw chemistry home uw organic chemistry home drawings produced with winplt. Amine pka = 38 ‐40 14. You'll never see a neutral nitrogen.
Source: www.masterorganicchemistry.com Using the pka values, one can see lactic acid is a stronger acid than acetic acid. Terminal alkyne pka = 25 13. Dempsey (eds.), ionization constants of organic acids in solution, iupac chemical data series no. Members of the basic family of amino acids, like lysine, will also exhibit three pka values. Ad aminozuren blends tegen betaalbare prijzen om je.
Source: www.chegg.com Amine pka = 38 ‐40 14. The ph at which the amino acid exists largely in a neutral, zwitterionic form (influenced by the nature of the sidechain) pk a~ 5 pk a~ 9 co2 r h h3n co + _ 2h r h h3n + co r h hn ho _ pka2 low ph high ph h3o _ pka1 table.